Chemical distribution
& Custom
Manufacturing


Customized Formulations
We specialize in crafting personalized chemical formulations, precisely tailored to meet your specific needs. Our team of skilled chemists and engineers collaborate closely with you to ensure the perfect alignment of properties and performance characteristics in our formulationsRead More...
01
Quality Assurance
Our foundation is built on quality, showcasing our unwavering dedication to the highest standards and safety. Rigorous quality control and cutting-edge testing facilities guarantee that each batch not only meets but surpasses industry and regulatory benchmarks, solidifying our reputation.Read More...
02
Process Expertise
Our skilled team offers extensive technical knowledge and experience. Refined processes ensure efficiency, yield, and quality optimization. From scaling production to resolving challenges, our experts guarantee a seamless, efficient, and cost-effective chemical manufacturing experience.Read More...
03
Cost Competitiveness
Our advanced technology-driven processes and supply chain management allow us to find cost efficiencies without compromising quality. With Elchemy, you get the best of both worlds – custom-tailored solutions at competitive prices. Read More...
04
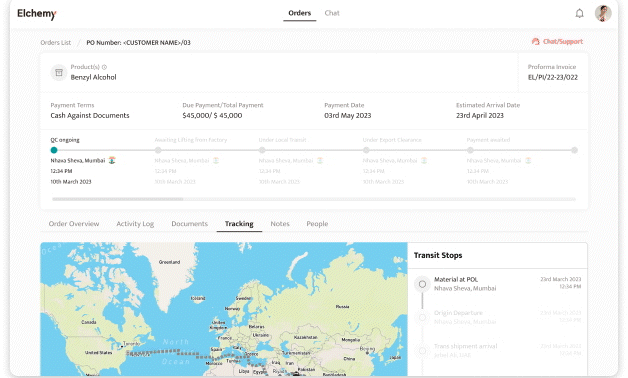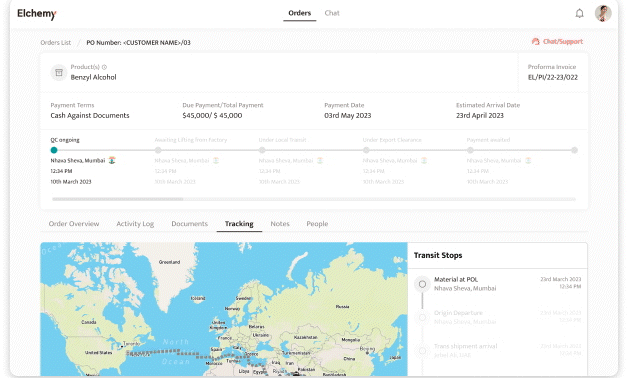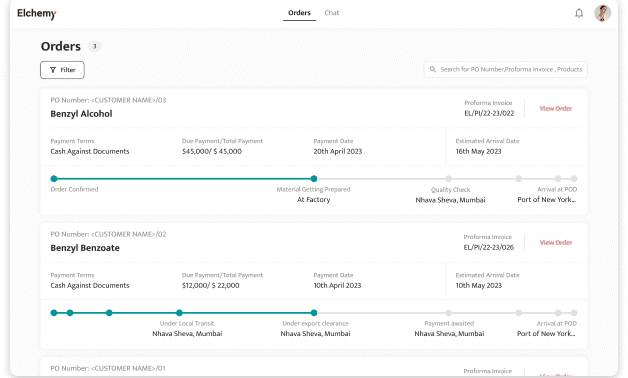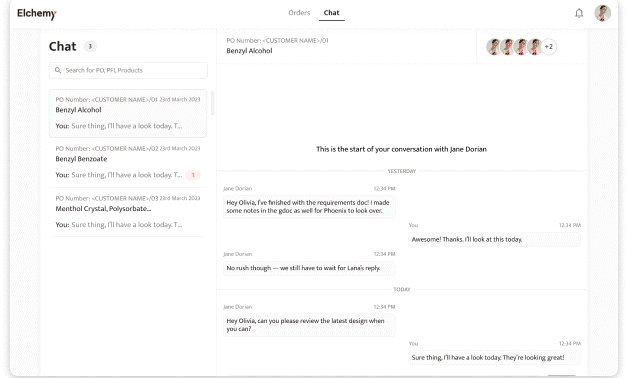
Crafted by Elchemy, this groundbreaking system is tailor-made to address the unique challenges of the chemical industry's operations. DOMS isn't just another software; it's your strategic ally in the journey towards operational excellence. Welcome to the future of supply chain management. Welcome to DOMSRead More...
We use cookies to enhance your browsing experience and provide personalized content. By clicking "Accept," you agree to our use of cookies. Read our privacy policy






